INCUBATING PROJECT | DECENTRALIZED TRIALS PLATFORM
Clinical Trials eConsent
Accessible, Convenient, Secure Trial Consent
The Decentralized Trials projects portfolio aims to transform the clinical trials industry by leveraging cutting-edge technology to streamline and digitalize the trials process using Verifiable Credentials, Decentralized Identifiers (DID), and product digital twins.
A decentralized and interoperable architecture can enable shorter, more efficient, and more accessible participation in clinical trials while improving monitoring and reporting, all of which can help reduce the time it takes for a life-saving product to reach the ones in need. Providing patients with a trusted and dynamic consent process for digital trial enrollment can make it easier for patients and providers to access clinical trials, while also reducing the administrative burden on trial sponsors.
Clinical Trials eConsent
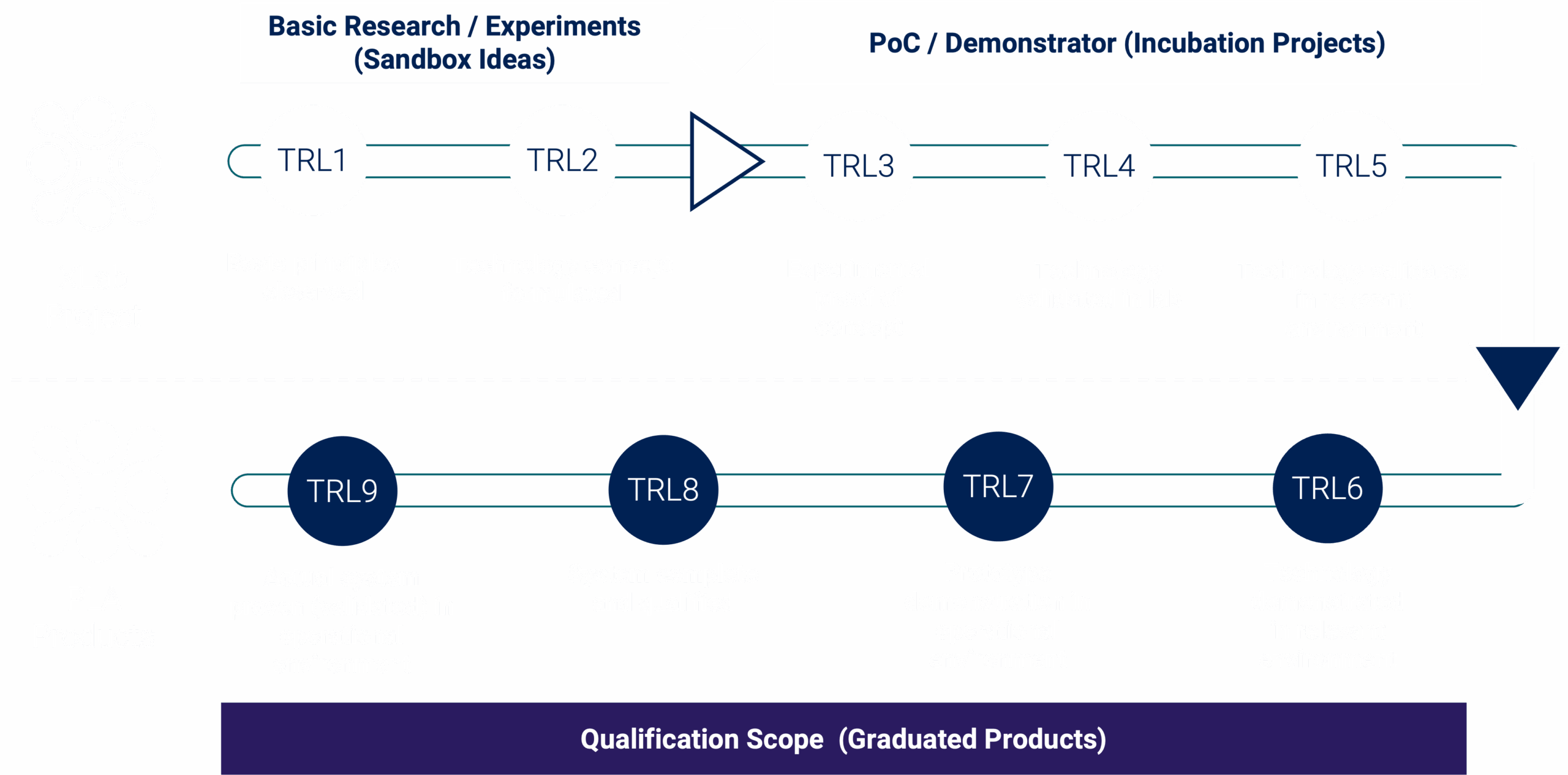
TRL Readiness
Technology Concept Formulated
TRL 2
Dive in this Project
Clinical Trial & Health Data Use Case Presentations
PharmaLedger’s 2nd Open Webinar
Blockchain-Powered Clinical Trials: Patient Centricity
PharmaLedger’s 5th Open Webinar
Joining PLA
As a Member Company:
By joining the PharmaLedger Association, you’ll become a key player in a collaborative network actively tackling systemic challenges.
As a Individual:
PLA is the venue for collaboration to incubate, develop, and launch Healthcare 4.0 products. While Membership is limited to registered entities individuals can also engage as contributors in the xLab without any fees.
Membership in PLA allows you to:
- Receive qualified and fully tested solutions
- Pool resources and risk with like-minded partners to realize increased benefits
- Promote your existing solutions within the PLA network
- Make decisions on the prioritization of our solution pipeline
- Operate a node on the PLA network to strengthen trust and resiliency

Join our PLA Collaboration Platform if you want to contribute contribute funding and expertise to the xLab program and have access to exclusive content in our xLab Strategic Working Groups.
Members, non-member organizations and expert individuals alike are all welcome.
All Rights Reserved © 2025 PharmaLedger Association








