On 6 August 2025, PharmaLedger Association hosted the first in a new series of open webinars, bringing together international participants and expert speakers to explore the intersection of digital and physical technologies shaping the future of pharmaceutical supply chains.
This session, titled “The Digital Pharma Chain: From Manufacturing Innovation to Secure Delivery,” offered a deep dive into practical solutions around traceability, security, digital twins, and packaging authentication.
Opening Remarks: Why Webinars Are Back
Stefan Schwarzfischer, Executive Director of PharmaLedger, welcomed attendees from across the globe and highlighted the importance of reconnecting the pharma community through open knowledge sharing. With the growing complexity of regulations, global cost pressures, and the rise in counterfeit risks, these webinars aim to foster transparent discussions and practical takeaways. He emphasized that PharmaLedger serves as a digital innovation hub and custodian of open, scalable infrastructure for the healthcare industry.
Presentation 1: Ali Badei, Gerresheimer
Topic: Digital Twin in Drug Delivery and Pharma Supply Chains
Ali Badei, Head of Digital Supply Chain Solutions at Gerresheimer, shared a compelling vision of how digital twin technology and blockchain can transform pharmaceutical manufacturing and delivery. With years of experience working on practical applications of digital tools in regulated industries, Ali brought technical clarity and strategic insight into how packaging can become a data-rich asset. He emphasized the power of combining unique product identification with decentralized data infrastructure to improve quality, traceability, and cost-efficiency across the product lifecycle.
Key highlights included:
Digital Twin Use: A digital replica of a physical product enables real-time traceability, quality control, and simulation of “what-if” scenarios.
Enhanced Traceability: By linking data from ERP systems, visual inspections, and quality controls, digital twins allow rapid root-cause analysis—potentially saving millions in discarded products.
Unique Identification & Blockchain: Each package or component is given a unique ID via 2D matrix codes or RFID, recorded in a decentralized blockchain ledger. This immutable identity framework strengthens security and supports traceability across databases while respecting data ownership.
Use Cases: From tracking contamination events to improving compliance, Gerresheimer’s digital twin approach promises greater transparency, efficiency, and cost savings—up to $40M annually for high-value drugs.
Success Factors: Ali emphasized the need for strong business cases, cross-functional collaboration, data governance, and scalability. He encouraged early engagement with regulators and partners to align implementation and expectations.
Speaker 2: Dr. Nadine Lamka, Schreiner MediPharm
Topic: Connected & Protected – Security Selection Criteria in Pharma
Dr. Nadine Lamka, Senior Product Manager for Pharma Security at Schreiner MediPharm, drew from more than a decade of experience in pharmaceutical brand protection and security consulting. Her presentation unpacked the strategic framework for selecting the right security measures to protect physical drug products against counterfeit and diversion threats. With a focus on practical implementation, Nadine emphasized that effective security isn’t about individual features—it’s about designing holistic, product-specific strategies that integrate physical protection with digital verification tools.
Her key points included:
Threat Landscape: Counterfeit drugs, gray markets, digital fraud, and packaging reuse all require unique security responses. Regulatory measures like serialization are not enough on their own.
Strategic Criteria for Security Solutions: Nadine introduced six key considerations for selecting security features: threat scenarios, verification target group, packaging concept, regulations, product characteristics, and solution feasibility.
Technology Overview: Schreiner MediPharm leverages a broad range of security technologies—covert, semi-covert, overt (e.g. holograms), and digital tools (e.g. NFC, smartphone-enabled authentication).
Holistic Approach: She stressed the importance of a “trusted entry point” into digital systems—where physical product integrity is essential to ensure that digital trust is meaningful.
Practical Guidance: Early involvement in product and packaging design, strategic tech selection, and stakeholder training are all critical to long-term success.
Closing Thoughts: Bridging Physical and Digital Worlds
The webinar closed with a key takeaway: digital trust starts with physical product integrity. Future-ready supply chains require a fusion of secure packaging, traceable digital identities, and collaborative ecosystems.
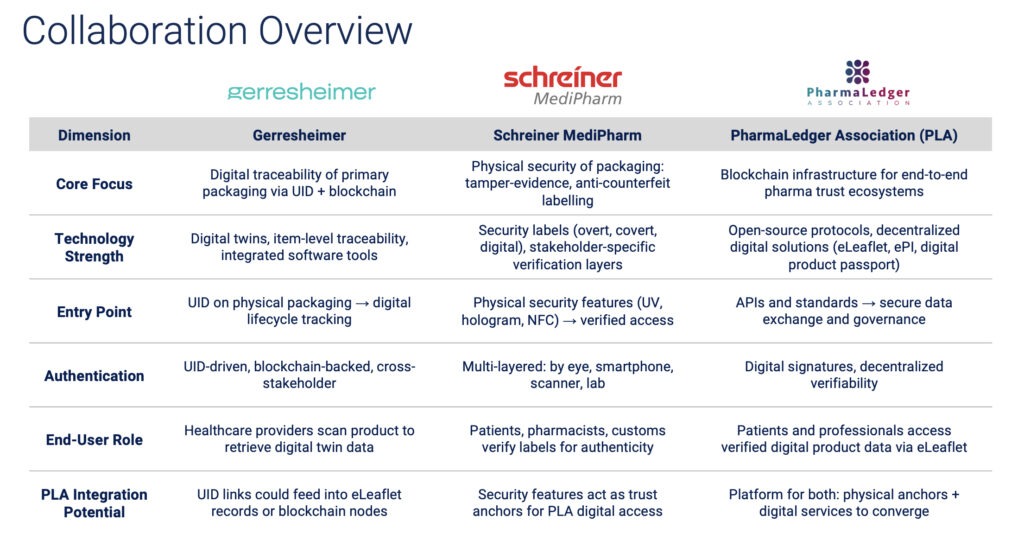
Gerresheimer, Schreiner MediPharm, and PharmaLedger Association (PLA) complement one another in building this secure and trusted pharmaceutical supply chain. Each organization brings a unique layer to the digital trust framework:
Gerresheimer focuses on digital traceability through UIDs (unique identifiers) and blockchain, enabling item-level tracking and digital twins.
Schreiner MediPharm provides physical security features like tamper-evidence, holograms, and NFC, ensuring that products can be authenticated at key checkpoints.
PLA supplies the open-source digital infrastructure—APIs, blockchain nodes, and eLeaflet systems—that connects physical products to verified digital data.
Together, this ecosystem enables end-to-end verification, where physical packaging, digital identities, and patient-facing information can interact seamlessly and securely. Here is how each partner’s strengths align across shared dimensions—from authentication to integration potential—creating a collaborative model for the pharma industry’s digital future.
Q&A Highlights
On Implementation Costs and Data Ownership: Ali explained that while blockchain infrastructure is relatively low-cost and scalable, databases should remain with individual stakeholders, ensuring secure and private access.
On QR Codes for Digital Product Passports: Nadine cautioned that QR codes alone are vulnerable. She advocated for secure combinations—QR + authentication layer—to ensure safety and trust.
On Regulatory Engagement: Both speakers emphasized early engagement, pilot projects, and participation in consortia (like PharmaLedger) as effective paths to influence and align with regulators, who may not dictate technology but care deeply about outcomes like compliance and patient safety.
PharmaLedger’s open webinar series will continue to spotlight emerging solutions and bring diverse voices into the conversation. If you’d like to propose a topic or speaker, the team welcomes your suggestions.
Watch the full recording on PharmaLedger’s YouTube channel
Subscribe to our newsletter to stay updated on upcoming sessions