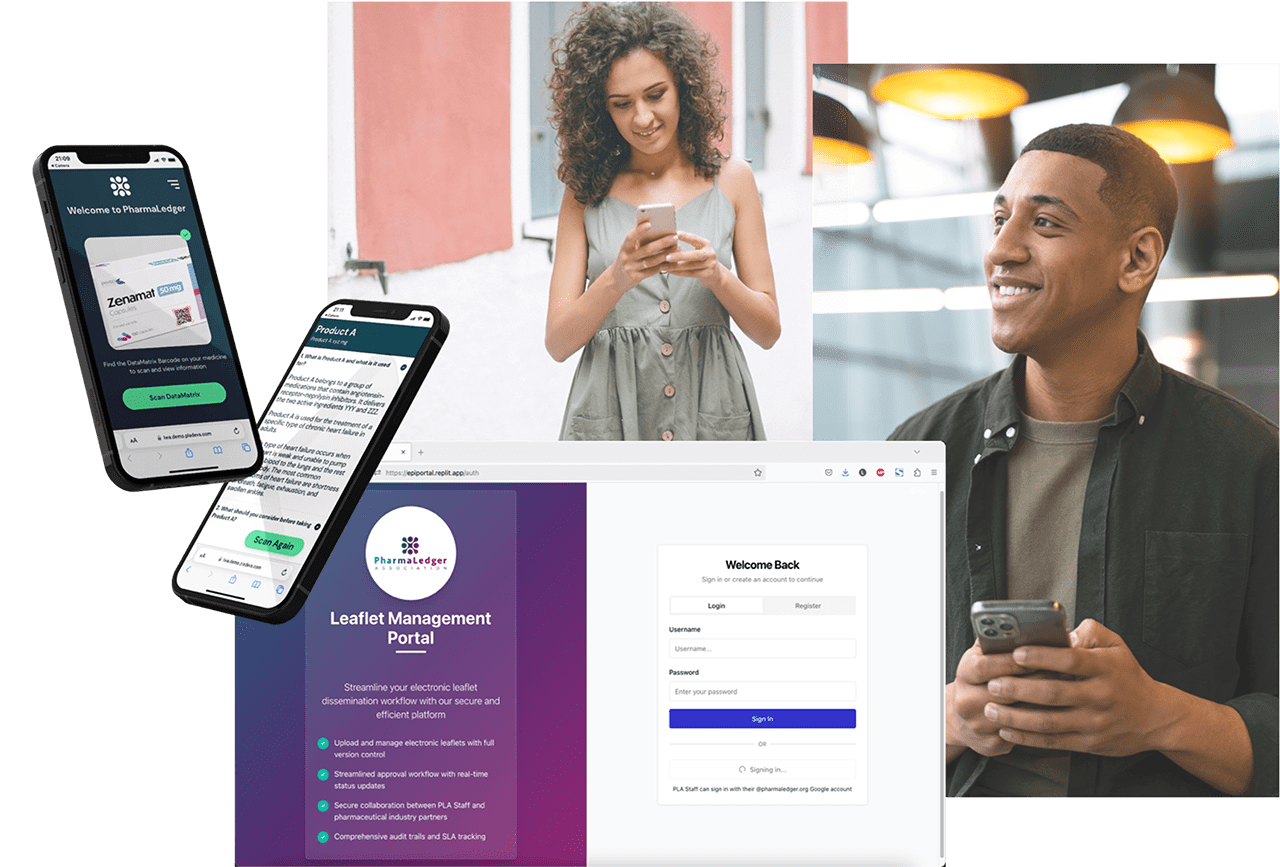
In 2023, PLA launched an electronic product information solution – ePI by PharmaLedger™ – developed with patients to deliver a user-centric ePIL experience which is easy to access and read. Powered by AstraLabel™, PLA’s scalable digital labelling platform, ePI delivers real-time SmPC, ePIL and labelling content directly to packaging, portals, and patient devices.
Digital Labelling Evolution Powered by AstraLabel™
ePI by PharmaLedger™
ePI by PharmaLedger™
Digital Labelling Evolution Powered by AstraLabel™

ePI by Pharmaledger™
Singapore, South Korea, Canada, Ecuador, UAE, Chile, Belgium and India are some of the countries already benefiting from the global standard ePI solution. 2025 expansion: Turkey and Brazil. Today, ePI by PharmaLedger™ is used by pharmaceutical companies to provide electronic product information to Patients and Healthcare Practitioners across the world, currently ensuring readiness in 46 countries and in 29 languages and serving as the cornerstone of the AstraLabel platform.

ePI by Pharmaledger™
In 2023, PLA launched an electronic product information solution – ePI by PharmaLedger™ – developed with patients to deliver a user-centric ePIL experience which is easy to access and read. Powered by AstraLabel™, PLA’s scalable digital labelling platform, ePI delivers real-time SmPC, ePIL and labelling content directly to packaging, portals, and patient devices.
Singapore, South Korea, Canada, Ecuador, UAE, Chile, Belgium and India are some of the countries already benefiting from the global standard ePI solution. 2025 expansion: Turkey and Brazil. Today, ePI by PharmaLedger™ is used by pharmaceutical companies to provide electronic product information to Patients and Healthcare Practitioners across the world, currently ensuring readiness in 46 countries and in 29 languages and serving as the cornerstone of the AstraLabel platform.
Access to the most current and approved product information
All features available in Portrait or landscape format for mobiles and tablets

LIGHTWEIGHT AND PRIVATE
MULTI LANGUAGES

TEXT TO SPEECH FUNCTIONALITY

FONT SIZE ADJUSTABILITY
Lightweight App that does not require download or sign in
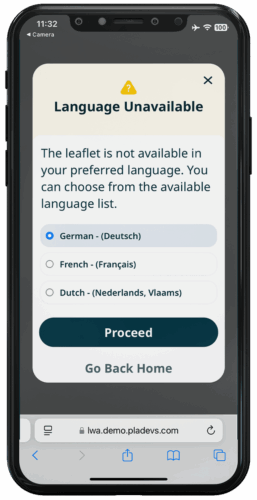
Available in multiple languages unlike paper leaflets
Our app supports text to speech to increase accessibility
The font size can be changed up to 300% for better reading
Access to the most current and approved product information
All features available in Portrait or landscape format for mobiles and tablets

LIGHTWEIGHT AND PRIVATE
Lightweight App that does not require download or sign in

MULTI LANGUAGES
Available in multiple languages unlike paper leaflets

TEXT TO SPEECH FUNCTIONALITY
Our app supports text to speech to increase accessibility

FONT SIZE ADJUSTABILITY
The font size can be changed up to 300% for better reading
Accessibility Compliant, Tailored for user, Paperless Ready
EPIL TYPE
PRINTING FEATURE
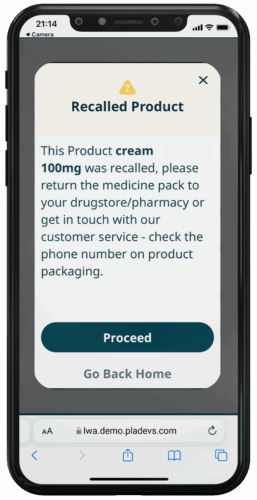
RECALL NOTIFICATIONS
For patients, a clear, easy-to-understand and user interactive. For healthcare professionals, quick access to pharmacological data and guidelines.
To promote equal access incorporating a printing feature is essential to bridge the digital divide
Recall Notifications prevents patients from continuing to use a potentially dangerous medication
Accessibility Compliant, Tailored for user, Paperless Ready

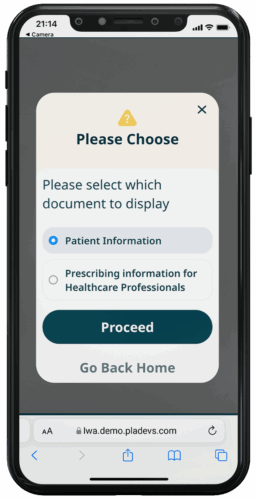
EPIL TYPE
For patients, a clear, easy-to-understand and user interactive. For healthcare professionals, quick access to pharmacological data and guidelines.

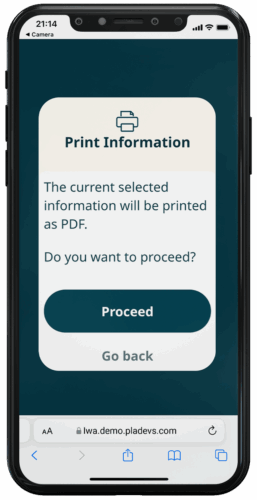
PRINTING FEATURE
To promote equal access incorporating a printing feature is essential to bridge the digital divide

RECALL NOTIFICATIONS
Recall Notifications prevents patients from continuing to use a potentially dangerous medication
AstraLabel™ & ePI by PharmaLedger™
Discover how they work together
From approval to patient in seconds.
ePI by PharmaLedger™ is the front end of the solution and included in any of the AstraLabel™ deployment models. Together, they deliver real-time SmPC, ePIL, and labelling content directly to packaging, portals, and patient devices.
Built for full regulatory traceability and zero-paper delivery, it supports pharma companies of every size with flexible deployments: Core, Solo, and Edge.
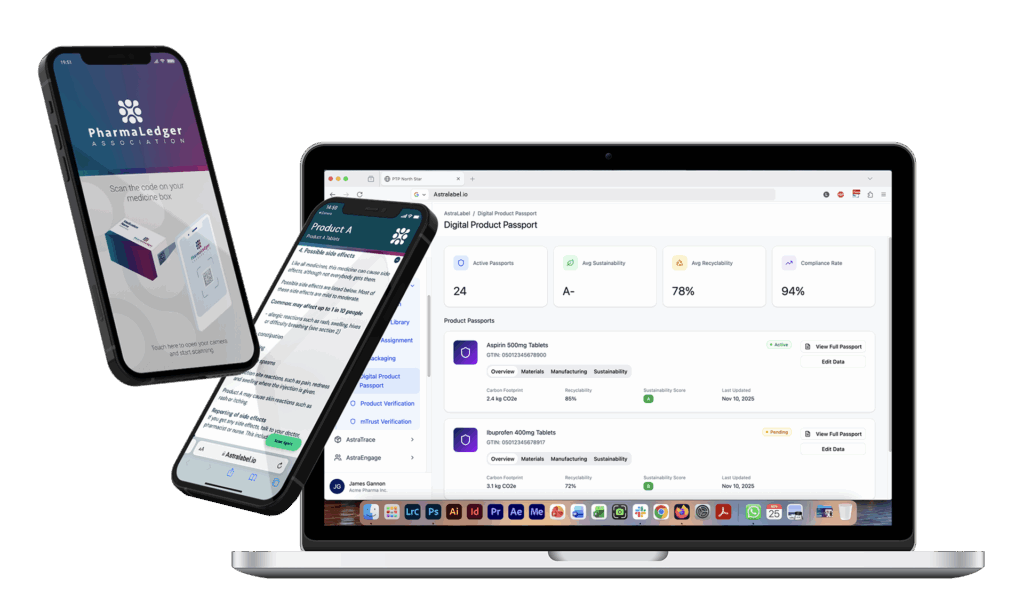

AstraLabel™ & ePI by PharmaLedger™
Discover how they work together
From approval to patient in seconds.
ePI by PharmaLedger™ is the front end of the solution and included in any of the AstraLabel™ deployment models. Together, they deliver real-time SmPC, ePIL, and labelling content directly to packaging, portals, and patient devices.
Built for full regulatory traceability and zero-paper delivery, it supports pharma companies of every size with flexible deployments: Core, Solo, and Edge.
Feedback Results | Patients
Data collected from Patients Surveys where participants rated their satisfaction on a scale from 1 to 5.
Access the full report here


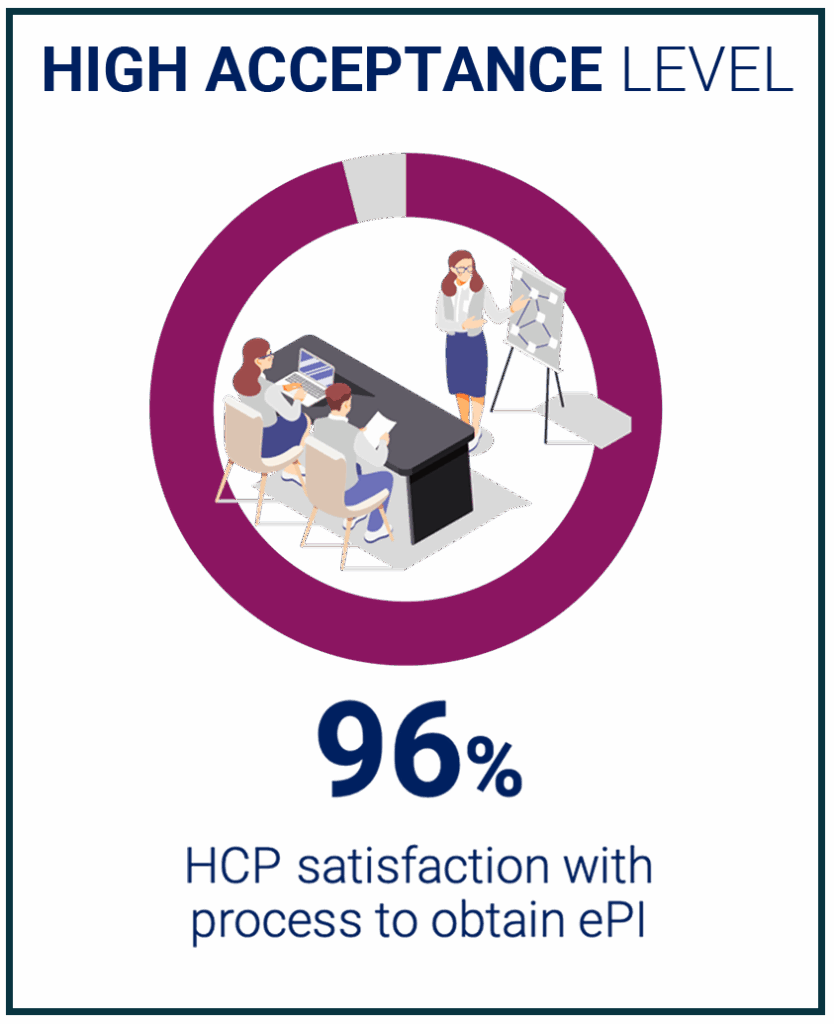
Feedback Results | Healthcare Professionals
Data collected from Healthcare Professionals Surveys where participants rated their satisfaction on a scale from 1 to 5.
Access the full report here



Feedback Results | Patients
Data collected from Patients Surveys where participants rated their satisfaction on a scale from 1 to 5.
Access the full report here




Feedback Results | Healthcare Professionals
Data collected from Healthcare Professionals Surveys where participants rated their satisfaction on a scale from 1 to 5.
Access the full report here




HOW DOES IT WORK?

ePI by PharmaLedger™ is accessible through a scan of the barcode on the product package. The scan reads the “GTIN” or Global Trade Identification Number and the batch and uses blockchain as a resolver to determine the correct MAH of the product.
The application then provides the patient or HCP with Product Information directly from the marketing authorization holder (MAH). The latest approved version of the Product Information is then provided to the user. The barcode on the product can also provide additional information such as the validity of the expiry date and serial number.
These can also be checked to verify the product packaging. It’s important that at no point is patient or user information captured. The patient’s use of the app to identify the product and access the information is private information. The product owner will not have any scan information to identify the user in any way, as this is private information.
HOW DOES IT WORK?

ePI by PharmaLedger™ is accessible through a scan of the barcode on the product package. The scan reads the “GTIN” or Global Trade Identification Number and the batch and uses blockchain as a resolver to determine the correct MAH of the product.
The application then provides the patient or HCP with Product Information directly from the marketing authorization holder (MAH). The latest approved version of the Product Information is then provided to the user.
The barcode on the product can also provide additional information such as the validity of the expiry date and serial number. These can also be checked to verify the product packaging.
It’s important that at no point is patient or user information captured. The patient’s use of the app to identify the product and access the information is private information. The product owner will not have any scan information to identify the user in any way, as this is private information.
Discover More about AstraLabel™ & ePI by PharmaLedger™
If you want to know more, please contact us. We look forward to finding the right solution for you and we are ready support your next step into the Digital Trust Ecosystem for healthcare.
All Rights Reserved © 2025 PharmaLedger Association


